
Preparing for MDR: Don’t Forget about Class I Reusable Devices
Learn about the new subclass for Class I reusable devices under MDR and the necessary steps to ensure compliance.

Learn about the new subclass for Class I reusable devices under MDR and the necessary steps to ensure compliance.

With the heightened supplier scrutiny, it’s time to reevaluate your supplier quality program.

A look at the practical implications of the regulations, the market opportunity and tips for compliance.
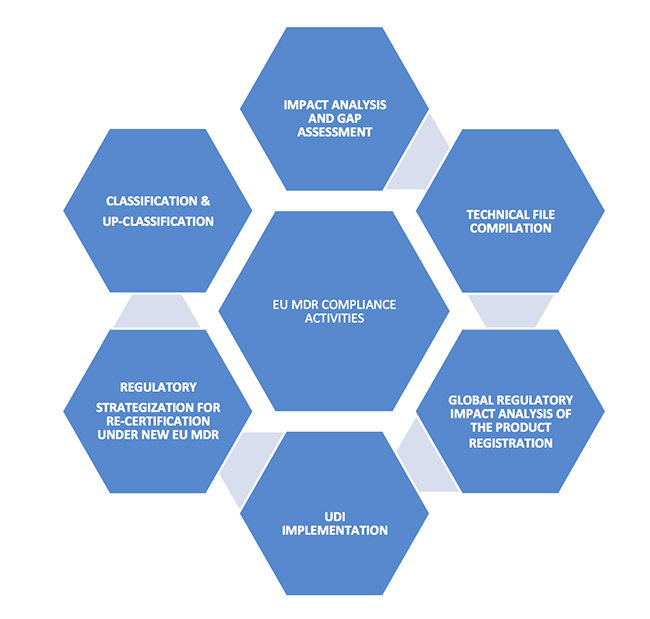
An overview of the key impact points and challenges of European Union Medical Device Regulation.

The company’s Bakken Research Centre in the Netherlands is centrally located for technology development and collaboration with physicians.

Critical subcontractors and crucial suppliers will be subject to unannounced audits by notified bodies under the revised European device regulations. Richard DeRisio of TÜV Süd provides more details in this presentation.

Annex XII (CE Marking of Conformity) of the Medical Device Directive is all about the Mark, the whole Mark, and nothing but the Mark. The regulatory gods in the EU like their CE marking of conformity just as the mark is depicted in Annex XII. Dr. D’s best advice is to abide by the Annex and leave perfection alone.

The takeaway from this edition of DG is extremely simple. Device manufacturers wishing to enter into the European device market must have a notified body. And under Annex XI of the Medical Device Directive, device manufacturers can take some comfort in knowing that notified bodies are also held to a high standard.

The takeaway from this week’s edition on MDD Annex X (Clinical Evaluation) is simple: the expectations of the Competent Authorities are that medical devices should always be safe and effective while conformity to essential requirements is achieved.

The take away from this week pertains to the accuracy of the declaration: device manufacturers need to ensure all of the information depicted within the declaration is factual and accurate, including those relating to rules and classifications.