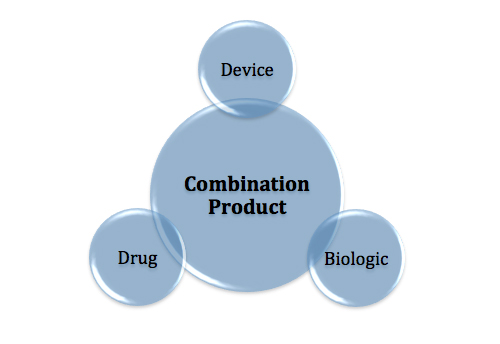
Combination Products: FDA Releases Guidance on CGMP Requirements
Manufacturers finally have some clarity on which CGMP requirements apply when producing a combination product.

Manufacturers finally have some clarity on which CGMP requirements apply when producing a combination product.

Some insights on integrating stability testing requirements into a device organization’s plan to make combination products.

The identification and traceability (sections 820.60 and 820.65) for products and finished medical devices, throughout the entire manufacturing process, including raw materials employed during the manufacturing process, and the subsequent sale and distribution of medical devices, are critical elements of the Quality System Regulation (QSR).

If a supplier scored well on their original selection assessment, or scores well on repeat maintenance assessments—say, a score of 90 percent or above for argumentative sake—and your organization is receiving 100 percent conforming product (yes, imagine a perfect world), what is the value of performing an annual or regularly scheduled maintenance assessment? Dr. D does not see any upside. Now I could write a philippic (yep, look up time again) that takes medical device organizations to task in regards t…

To ensure supplier surprises are kept to a bare minimum, an organization cannot simply rely on thaumaturgy (go ahead and look it up). Supplier performance must be continuously gauged for effectiveness, and a feedback loop created to ensure suppliers are receiving accurate information in regards to their overall performance. Additionally, supplier performance metrics should be included into your management review process. Management must be informed of parlous issues that can result in the need for field…

Welcome back to this sixth installment of Devine Guidance . I am not one to assume a Panglossian view (time to pull out the dictionary again) when dealing with problem suppliers and the need to pursue corrective action. However, at times the ugly SCAR becomes unavoidable. In fact, evidence of effective corrective action, as it pertains to suppliers is a requirement and the FDA, notified bodies, and other regulatory bodies will verify the effectiveness of the approach pursued by your organization. Additi…

Since the concept of writing a weekly blog and actually having a following is somewhat foreign to me, I was not sure what to expect or the reception I would receive writing a weekly column. So far, the support and responses have been overwhelmingly positive; and for that, I would personally like to thank the readers. Additionally, I will continue to write about and provide relevant information associated with supplier quality (four installments remaining) and will eventually begin addressing quality and…

If you are a returning visitor, welcome back to this 4th installment of Devine Guidance. If this is your first time, I hope you have a chance to read the first-three installments of my blog. In this installment, I will continue with the defensive-receiving inspection theme, a process that I feel “has limited-value.” That said,…

To those who have decided to return for another episode of Devine Guidance , thank you! Once again, I hope you find some reading enjoyment and value in this third installment. For this adventure, I plan to dive into the importance of a clear and concise specification to support the design, development, and procurement processes.

To my colleagues who have decided to rejoin me for an ongoing exploration into the trials and tribulations of effective supplier management for the medical device industry, thank you for returning. I hope you will be able to glean some useful information from the compilation of current industry practices, concepts extracted from my doctoral dissertation, and an overall common-sense approach to supplier quality. It is my personal belief that pursing the ideas presented within this paper, and scheduled to…