
FDA Announces Cybersecurity Public Workshop
The agency will bring together major stakeholders to discuss challenges in medical device cybersecurity.

The agency will bring together major stakeholders to discuss challenges in medical device cybersecurity.
When preparing a regulatory submission, there are a couple of critical elements to consider.

If you haven’t made the connection yet, you’re in trouble.

Leveraging big data and clinical evidence is top of mind.

As the agency continues to try to do more with fewer resources, taking additional risks is imperative to move forward.

CDRH’s Carl Fischer shares the agency’s approach to organizing and managing complaints.
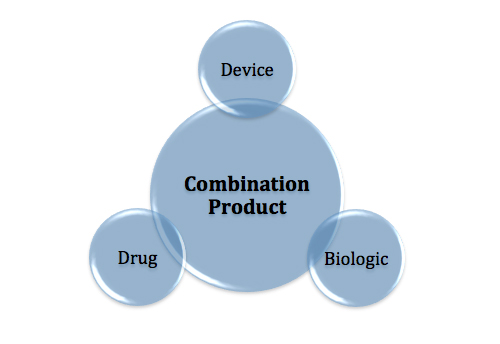
The creation of the Office of Combination Products more than a decade ago may have been a big step forward, but frustrations surrounding policy-making and coordination between CDRH, CDER and CBER remain.

This year’s work plan includes several items that will impact medical device manufacturer and suppliers.
The last decade has brought more interaction between the device industry and FDA related to conducting health hazard evaluations, but companies continue to face obstacles.
The last decade has brought more interaction between the device industry and FDA related to conducting health hazard evaluations, but companies continue to face obstacles.
From ensuring patient safety to protecting a company’s reputation, the product recall process starts with putting together the right team of qualified individuals.
From ensuring patient safety to protecting a company’s reputation, the product recall process starts with putting together the right team of qualified individuals.