According to the World Health Organization, cervical cancer is the fourth most common cancer in women worldwide and the second-most common cancer in women living in less developed regions. Its number one cause is the human papilloma virus (HPV), which also happens to be the most common sexually transmitted infection in the United States as well as globally. Since it can take up to 10 years or longer for precancerous lesions to develop into cancer, early detection is key; when cervical cancer is caught in its early stages, it can be treated with success.

In the developing world, access to affordable technologies that enable early cervical cancer detection is not widespread. Imagine having the ability to not only provide such a solution but also do so using a mobile platform. It would have the potential to change the healthcare landscape, and more importantly, save lives. One might think that this type of technology is already being taken for granted in the United States, but in this case, this type of platform could truly change the standard of care in detecting precancerous changes both in the United States and throughout the world.
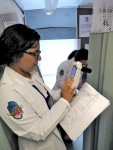
One small start-up company based in Israel is positioning itself to change the standard of care in cervical cancer screening and detection, and in an impressive way. MobileODT has developed a technology that leverages a smartphone, biomedical optics and cloud-based software for screening and detection at the point of care (think Haiti, East Africa, and Nepal, to name a few places where the platform can be used). “Our goal is to make diagnostic imaging available to everyone on the planet,” says Ariel Beery, CEO of MobileODT. “We believe that with many diseases, especially ones that are so easily treatable, such as cervical and oral cancer, and anal dysplasia, if you catch them within the five-year window of HPV infection, you can treat them on the spot.”
The company’s approach has been to build medical devices that are inexpensive enough to become ubiquitous, yet so simple to use that even those with low levels of training could still effectively conduct screening. “We developed a technology that would enable us to significantly lower the cost of delivering high-grade advanced optics into any environment in the world,” says Beery. The device platform supports the entire cervical cancer screening workflow and in rural areas, eliminates some of the subjective element within the process, which is essentially analysis with the naked eye. “What one woman sees with her eye and a flashlight is very different from what another woman sees with her eye and a flashlight,” says Beery, indicating how the process works in the developing world. “By connecting a light source to the cellphone and the camera, along with the magnification that it enables, we can help standardize the image and provide a significantly better view of the cervix.” During the screening process, visual inspection takes about two to three minutes, followed by about one minute to review and make an assessment.
The technology’s software and networking capabilities allow clinicians to consult with others when making decisions. “At the same time that they’re using the device and collecting data, we’re collecting data,” says Beery. Copies of the images are put into MobileODT’s research database, which enables the company to build a pattern, learn from them, and apply a big-data approach to image processing and analysis. “This will help us gather a better sense of what’s happening in terms of their screening and from there, build a set of diagnostic algorithms that will enable us to later support the clinician at the point of care with an automated decision. We believe that worldwide, it will bring significant value in catching diseases at earlier states by correctly understanding the risk factor associated with each particular case based on the models that we’re able to generate.”

Founded in 2012, MobileODT already had its first prototype in the field by April 2014. However, the process was obviously not without hurdles. “For us, the biggest challenge initially was to identify the right way to get an accessible, inexpensive and powerful conduit for our IP and provide support for the screener on the ground,” says Beery. “Then we needed to build a product that fits into their everyday workflow, and is robust and durable enough to survive varying conditions—from Haiti’s humidity to Kenya’s dry heat to Nepal’s cold and high altitudes.”
By April of this year, MobileODT released its first product to the European market after receiving the CE mark. It has sold 108 units, which are being used in 16 countries, with orders currently exceeding the company’s wait list. According to Beery, MobileODT is working toward a mass manufacturing capability in order to meet the 1000+ requests for the product. The greatest adopters of the device, which is currently manufactured in Israel, are private nurses who run clinics in East Africa.
The company is also one of four finalists in the MedTech Innovator 2015 Competition. Selected out of 300 companies, MobileODT and the other finalists had to demonstrate their ability to deliver improved value to the healthcare system. “For years, we’ve heard the pitch for small, portable and powerful medical devices that are networked and can change healthcare, but it hasn’t been compelling,” says Paul Grand, managing director for RCT Ventures and head of MedTech Innovator. Now, industry is seeing a convergence between the strong demand to demonstrate both the advantage to patients and the ability to benefit the overall healthcare system. “It’s a powerful technology with a network and the ability to link data,” says Grand. “They’re going to store every image and be able to predict things in the future. We’re all looking for technologies that are actionable and look at data behind the devices. His [Beery’s] company is delivering in that area. It’s not just a mobile phone with a lens.”
The selection committee for the MedTech Innovator competition was not just pleased with the company’s platform and its ability to provide it at the right price point, says Grand. They were impressed with the fact that MobileODT has been able to deliver in the harshest of environments—the developing world. “And they’re doing it at a time in which it’s challenging to do so, in terms of the company’s size. They’re executing incredibly well.” The winner of the MedTech Innovator competition will be announced next week at the 2015 AdvaMed conference.
For the next six months, MobileODT is focusing on the cervical cancer application. However, it is also conducting pilot programs to use the technology for applications in forensic colposcopy (sexual assault documentation), oral cancer, and anal dysplasia.
Update: October 7: MobileODT has won the 2015 MedTech Innovator Award.