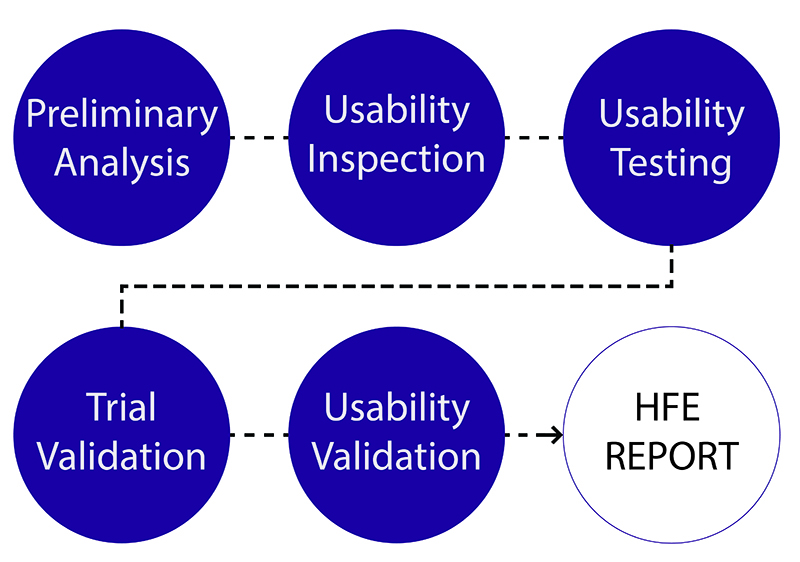
There’s some good news for manufacturers of combination products that deal with the confusion surrounding whether their product will be regulated as a device, drug, biologic or combination product. FDA is revamping its internal procedures and has a draft guidance in the works about the Pre-Request for Designation (RFD) process.
In a blog on FDA Voice yesterday, FDA’s Director of the Office of Combination Products (OCP) Thinh Nguyen, and Rachel E. Sherman, FDA associate deputy commissioner for medical products and tobacco, explain the concept of the Pre-RFD process and its comparisons to the RDF process: “In both cases, FDA’s assessment depends on sponsors providing a complete, clear, and detailed product description, which includes the product’s indication for use, its composition/ingredients, and an explanation of how it works. In most instances, both processes also require input from the product jurisdiction officers in the relevant Centers and, if necessary, legal perspectives from the Office of Chief Counsel.”
The Pre-RFD process is more interactive than the RFD process and can be used at anytime during product development. The goal is for sponsors to receive a response from OCP within 60 days. According to Nguyen and Sherman, sponsors can benefit from the Pre-RFD because they’re not required to recommend classification and assignment of their device with corresponding rationale nor do they have to discuss the classification of products currently on the market and how their product is similar. Sponsors can also get preliminary feedback from FDA via this process.
FDA is currently putting together a draft guidance about the Pre-RFD process that will include information about what details sponsors should have in a Pre-RFD and how FDA will review it.