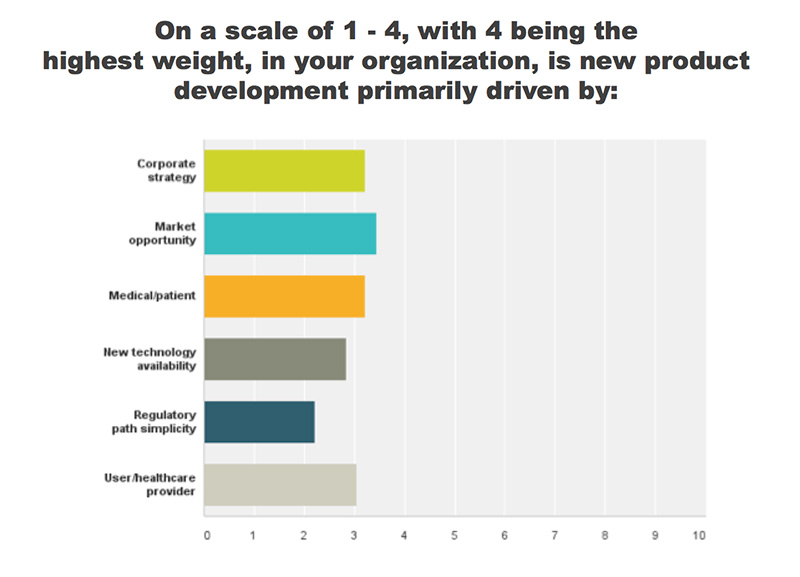
REGISTER HERE to attend the MedTech Product Development Conference | September 29-30, 2016 | St. Paul, MNSeveral key factors drive product development. In a recent MedTech Intelligence survey, respondents identified market opportunity as a leading driver, closely followed by patient needs, corporate strategy and healthcare provider need. While these aren’t surprising factors, some companies can get ahead of themselves during the product development process, letting certain elements cloud the pathway. “We really need to remind ourselves that it starts with the user and that the technology is the enabler—it’s not the core function,” said Craig Scherer, co-founder at Insight Product Development. During a roundtable discussion, industry experts shared the following insights on identifying users and the upfront challenges that medical device companies experience.
MedTech Intelligence: What are the key challenges that device manufacturers face in identifying user needs?
“Three areas drew my attention: Scope creep, time and resources, and the need to prioritize,” said Nolan Baird, CMC Director, Combination Product Development, Drug Product Development, at AbbVie.
- Scope creep: What are the must-have user needs versus the nice-to-have user needs, and what are the tradeoffs? “We struggle quite a bit with nailing those down and having a firm understanding of what’s an absolute requirement versus what is something that isn’t necessarily required,” said Baird.
- Having the time and the resources to do good research. “I emphasize the word ‘research’,” said Baird. “We are a research and development organization, but we sometimes forget to do that research upfront and invest the time and resources to truly identify and understand the user needs from a program early on when it’s most critical.”
- Prioritizing various user needs. This includes identifying the needs and prioritizing them to make sure the focus is on the right aspects and that there’s a balance with the business drivers for both the development program and the organization. “Quite often you get user needs that can be extravagant and expensive and have to be weighed against the other business drivers as far as cost and present value of programs,” said Baird.
Baird stressed the criticality in defining users. “Before we can understand the needs we have to understand our users,” he said. “What we’ve found on a couple of recent programs is [that] quite often, for various devices and delivery systems, there are users above and beyond just the patient or the healthcare provider—the whole patient experience, or in the hospital, supply chain, between payers and insurance companies, and even stakeholders within an organization—there are various needs of various users on so many different levels. It’s important to cast that broad net to not only capture all the different needs but even a step further back to make sure you capture all those potential users and influencers.”
According to Eric Soederberg, president and CEO of Sunrise Labs, convincing management of the importance of user needs is a challenge. “Having management sometimes believe they already know what the user needs are can be a problem. We see that mostly at smaller companies; larger companies seem to have managers that have been through it before and understand the value,” he said.
Soederberg also emphasized the need for companies to focus on the most viable markets. “To try to be everything to everyone—the number of user groups that [companies] have to study and the number of different emergency rooms, state labs, doctor’s offices, patient homes, etc.—if they try to do everything, it’s unlikely that you’ll be able to make a path that satisfies or that’s good enough for each constituent. That’s the other challenge that folks have: To focus properly, using whatever business intelligence they have to choose the right one first.”
“The lesson that’s important for people to pick up on and learn as quickly as possible is to cast a broad net—try to get as much as you can from that user group,” said Jon Speer, founder of greenlight.guru. “I think Eric said it really well: Understand the specific market you want to dive into and focus on that market, and try to be as comprehensive as you possible can.”

Scherer focused on a few areas in which companies need to spend adequate time—understanding, characterizing, and prioritizing user needs. “Make sure you spend enough time not just to satisfy formative and summative evaluations but also do discovery research and use those evaluations to drive through better design,” he said.
And the final word of caution: Avoid falling to the Shiny object syndrome. “This can unfortunately be a consequence of mergers and acquisitions. These great new technologies come out, and it seems like an elegant solution to go after some of these new technologies,” said Scherer. However, the problem is that these technologies may not actually meet the user needs correctly. Scherer advised that companies evaluate and characterize the user needs first before moving forward with refining a technology in this situation.
Hear more of the roundtable discussion: “MedTech Product Development: Getting the Right Products to Market Sooner”.
In the coming weeks MedTech Intelligence will share additional results of its product development survey. Learn more about the upcoming MedTech Product Development Conference here.








