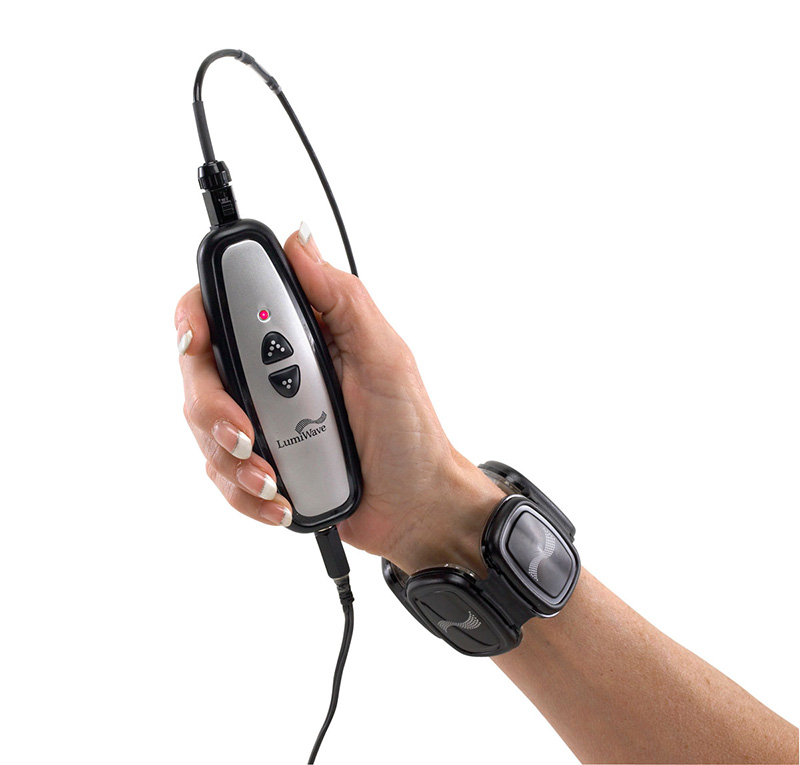
As medical technology continues its rapid transition into the home, the challenge for device companies lies both in designing products that are easy to operate as well as affordable. When it comes to over-the-counter devices within the physical therapy space, patients have a variety of options, from heat wraps and transcutaneous electrical nerve stimulators to infrared light therapy. Prices can range from $40 to more than $1000, and many products are not entirely effective in pain management. While there isn’t a cure-all for treating aches and pains, and even fractures, one company is going after the home healthcare market with a device that uses infrared light therapy to promote natural healing and long-term regeneration.
BioCare Systems, Inc. is officially launching the device, called LumiWave, to the public tomorrow at the Consumer Electronics Show. Touted as the first LED-powered medical-grade device for over-the-counter use, LumiWave is designed to help heal inflamed tissue through 20-minute treatments of infrared therapy twice a day. The infrared light heats the affected area locally and stimulates circulation. It may also be a safer option versus other pain management therapies such as Cortisone shots and medication that is taken over the long term.
Although the FDA-cleared device has been available for more than 10 years, the company has been conducting extensive market research to refine the product in an effort to move it out of the clinic and into the home. “We were chasing the market as it was starting to mature,” says Jon Weston, CEO of BioCare Systems. “Now the market has gotten to the point with attitudes about healthcare and out-of-pocket expenditure that everything is aligning for this kind of device.” Through the market research, the company identified two core groups of available therapy products. The first group contains five to 25 LEDs and costs between $75 and $99. According to Weston, these products don’t put out enough energy to be clinically effective. The second group was devices with 200-400 LEDs, which tend to be used in physical therapy offices, clinics and athletic training rooms, thus garnering a price tag of $4000–$6000. “They’re very effective but not attainable by the consumer, if you can even buy them—most are [only available by] prescription,” says Weston.
The design of the latest generation of the device was partially driven by the goal of making it more affordable for home use. BioCare Systems went through three rounds of product design. The first round involved the fundamentals—ensuring that the LEDs could effectively deliver light energy at the right wavelength (the company’s core IP surrounds the technology itself: the electronics, LEDs and light modulation). During the second phase, the device was tested by consumers, physicians and trainers, and further refinements were made with human factors in mind, including making the device portable and more rugged yet flexible so it could be used on several different areas of the body. While it was clear that Olympic professionals and elite athletes liked using the device, the company needed to drive down the price. This third round of design took about four years, according to Weston. “How do we keep current quality and performance, yet reduce the cost of design by perhaps combining certain electronic components?” says Weston, adding that the device has more than 230 components. After speaking with several contract manufacturers about taking the design and driving down the cost of the components and the finished device, BioCare Systems settled on Arciplex, a firm located in Nashville. Through the partnership, they were also able to keep 65% of the production and fabrication in the United States, allowing the device to contain a Made in USA label.
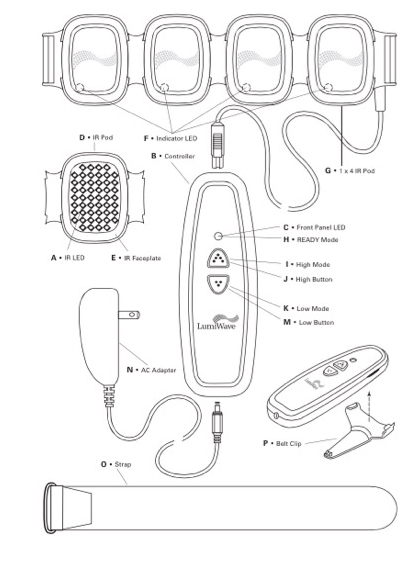
Consumers can purchase the 200-LED version of the LumiWave with four pods (50 LEDs on each pod) for $299 or for more coverage, a 400-LED version that has 50 LEDs contained on eight pods for $449. And like most companies, BioCare Systems is already looking at what’s in store for the next-generation of the product. Modifications include a battery-powered unit with a charging station (the current device must be plugged in, thus the user is tethered and cannot walk around) and a car charger unit.
The company also plans on exploiting more of its FDA claims and patents, the first of which will be a dental mouthpiece for treating gum tissue (BioCare owns a patent for using light to treat dental tissue), namely for people who have periodontal disease. Currently in design, the product would look like an athletic mouthpiece with embedded LEDs that illuminate the interior of the mouth. “Certain wavelengths of near infrared light trigger cell repair mechanisms in the body, causing release of a chemical called nitric oxide, which increases blood flow,” says Weston. “It’s a signaling molecule for repair and healing processes. Any time you have tissue that isn’t performing well or is damaged, near infrared light, through nitric oxide, turns on these repair mechanisms to bring the tissue back up to healthy level.” The device, which would have a four- to six-month lifespan (for hygienic reasons), would be used for 20 minutes a day. Weston anticipates submitting a 510(k) for the product later this year, potentially receiving FDA clearance some time next year.
The company also plans to conduct additional clinical studies on accelerated clinical healing, both for soft tissue healing in ankle sprains and fracture healing. “We can accelerate fresh fractures (normal fractures) in two to three weeks in healing rather than six to eight weeks in healing,” says Weston. He anticipates receiving submitting a PMA for this application by year-end or early 2017.