Global regulatory bodies allow the commercial release of product following all appropriate testing and clearances, with accelerated aging data. This compromise allows manufacturers to bring life-saving devices to market quickly based on the proven Arrhenius model. However, the model cannot be certain to capture all material interactions and combinations of stacked challenges. Therefore, most regulatory bodies require that real-time aging accompany accelerated aging testing to ensure alignment and agreement of the two groups and the veracity of the Arrhenius method for each unique product.
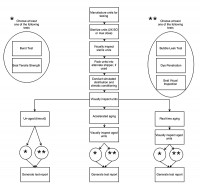
At the same time as the accelerated aging units are placed into ovens, place the 13- and 25-month real-time aging product in a temperature- and humidity-controlled and tracked area under ambient conditions until the required time(s) have elapsed. Upon the conclusion of the elapsed time, remove the product and test the seal strength, seal integrity and product function. Compare these results to the results obtained for un-aged product, in order to identify any existing trends in stability or degradation of the product or packaging. Further, compare the test results of the real-time aged units to the test results of the accelerated aged units in order to ensure the real time aged units will perform acceptably in the field.
This test progression can be more simply visualized with a map as seen in Figure 1.
Once all the testing is completed and the data are analyzed, you are ready to write the DV test report. Remember to include all raw data sheets, training records, and data analysis, including normality testing, in the report. It is generally advisable to release multiple DV reports, one for each period of accelerated and real-time aged product. You may report product functional testing separately as well. There are no specific requirements for the contents of the DV reports in terms of aging periods or packaging versus product results—this is a function of convenience and practicality for the manufacturer to determine. You may encounter a critical reporting circumstance if non-conformity is discovered during testing. Unless the data can be definitively shown to be the result of an assignable cause (e.g., transcription error, improper loading of the package into the sealer, etc.), then the company must be ready to determine the impact and potential necessity for a field action or other remedial action. For this reason, it is recommended that a notebook study be performed prior to the DV testing in order to characterize any potential shortcomings of the design of the package, the product, or the interaction of the two. By the time you get to DV testing, there should not be any surprises.
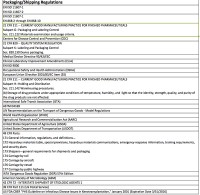
There are myriad regulations and standards that require and prescribe design verification testing of packaged product. Some of them are included in Table 3.1
Packages must be able to withstand the typical events associated with distribution of the product without defect or loss of sterility. Manufacturers are responsible for evaluating and documenting the package’s ability to protect the product throughout handling, distribution as well as within the storage environment.2
However, perhaps the most compelling reason to perform DV testing, especially with the stacked challenges recommended herein, is the potential for discovering untoward post-market outcomes before they cause harm to the patients whose health is our responsibility. Clearly, anything less is not only unethical, but invites unwanted product liability actions, potential damage to a manufacturer’s good name, and in the event of a field action, erosion of market share.
References
- Goode, R. D., (February 2003). “Planes, Trains and Automobiles: Simulated Distribution of FDA-Regulated Products for Packaging Validation,” Journal of Validation Technology, Vol. 9, No. 2, pp. 181.
- Distribution Dynamics Certified Testing Laboratory. “Package Testing, Product Testing and Materials Testing for the Medical Device Industry”. Retrieved from: http://www.testedandproven.com/