The pubic views tissue engineering as the growing of tissues and/or organs in a pristine, sterile white lab with vapor spewing from large vats, with beautiful people scurrying about in lab coats with petri dishes, as depicted in Hollywood movies. Although this may be possible in the distant or perhaps intermediate future, the reality of today is much different and of even greater potential. The first baby steps into clinically practical tissue engineering may be viewed through in-situ tissue engineering of bone.
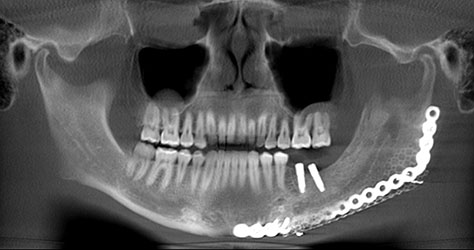
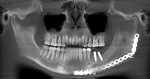
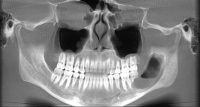
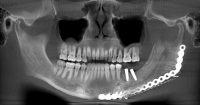
What we call in-situ tissue engineering of bone is the regeneration of viable, functional bone, capable of remodeling and growth at the point of care in a hospital operating room. In the head and neck areas, and particularly in the jaws, we have regenerated 6 cm or more of bone (without any open bone harvesting) in more than 1,000 patients.
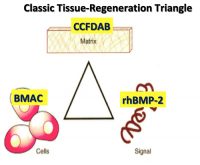
The concept of in-situ tissue engineering of bone is based on the classic tissue engineering triangle of cells-signal-matrix (see Figure 1). Using three specific medical technology advances for each and combing these three elements have proven to be safe and predictably effective.
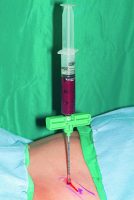
Cells. The Mesenchymal Stem Cells/Osteoprogenitor Cells (MSC’s/OPC’s) are obtained at the point of care by either of two technologies. Both harvest the true mesenchymal stem cell CD34+ as well as the MSC’s/OPC’s CD44+, CD90+, CD271+, and CD105+. The first is the Harvest Technologies system, which aspirates 60 ml of autologous bone marrow from either the anterior or posterior ilium. Using four separate punctures (two on each side), each aspirates 5 ml of Bone Marrow Aspirate (BMA) using a heparin-coated 20 ml syringe (see Figure 2). Each 15 ml of BMA is then added to a transfer bag containing 4 ml of ACDA anticoagulant. This 60 ml total is then removed from the transfer bag and placed into a proprietary canister that contains a floating shelf, separating the MSC’s/OPC’s by differential gradient density from the bone marrow plasma (see

Figure 3). The specific centrifugation protocol uses a 14-minute double spin cycle after which the bone marrow plasma is separated from the MSC/OPC bag, yielding a Bone Marrow Aspirate Concentrate (BMAC) containing a four-fold to six-fold increase in the MSC/OPC cell count.
The second technology is the Lenkbar Marrow Marxman which also aspirates BMA. However, because of its patented flexible design and increased strength, it can safely scythe along the inner cortical surface where the greatest number of MSC’s/OPC’s reside (see Figures 4, 5, and 6). With this system, only 10 ml of BMA is needed, which contains as many MSC/OPC’s as the BMAC without requiring a centrifuge and without requiring an off the sterile field manipulation.
|
|
|
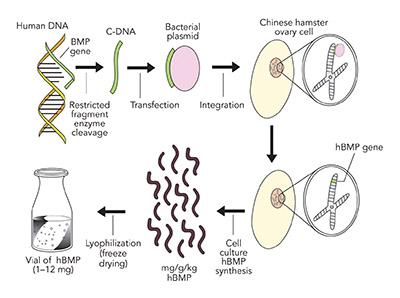
Signal. The proven signal for bone regeneration is recombinant human Bone Morphogenetic Protein-2 (Acellular Collagen Sponge –rhBMP-2/ACS–Infuse Bone Graft Medtronic.). BMP-2 is a protein coded from chromosome 20 in the human genome. This gene sequence is separated from the chromosome by restricted fragment enzymes, which is then attached to a bacterial extra nuclear ring of DNA known as a plasmid. This plasmid, carrying the BMP-2 gene sequence, is then transfected into a Chinese Hamster Ovarian (CHO) cell in culture, which produces a number of hamster proteins but also a large amount of a single human protein, BMP-2. The human BMP-2 is separated from the hamster proteins by electrophoresis and nano filtration to yield a 98.8% pure rhBMP-2, which is freeze dried into a white crystalized powder for clinical use (see Figure 7).
The clinician selects any one of five doses, 1.05 mg, 2.1 mg, 4.2 mg, 8.4 mg, or 12. mg, and dissolves the freeze dried crystals in the pre-set packaged amount of sterile water so as to gain a concentration of 1.5 mg/ml, which is then placed on the acellular bovine collagen sponge and allowed to bind to the collagen for 15 minutes before use.

Matrix. The matrix is derived in part from the BMAC or Marrow Marxman plasma component, which contains the cell adhesion molecules fibrin, fibronectin and vitronectin as well as the stem cell activation component of stromal derived activation factor 1-alpha (SDAF-1 alpha). These molecules are strands that interlock and adhere to collagen on bone surfaces. They will act as a scaffold upon which osteoid can be deposited and mature bone formed.
The second portion of the matrix comes from mineralized Freeze Dried Cancellous Allogeneic Bone (FDAB), produced by a number of tissue banks registered with and adherent to the guidelines of the American Association of Tissue Banks (ATB) (see Figure 8). In the case of such cadaveric donors, bones are harvested within 24 hours of death or less and only after a complete medical screening for disqualifying conditions or contamination. Various bones are harvested under strict operating room sterile technique and are cultured. The bones are cleaned of any adherent soft tissue, then are high pressure washed to eliminate all cellular components that may pose an antigenic stimulus before being held in a liquid nitrogen
Once all medical screening, culture testing and serology has certified that the harvested bones as derived from a safe donor and are free of transmissible agents, the bone specimens are lyophilized (freeze dried), which amounts to removing all water in a vacuum over 21 to 35 days. The finished product used for in-situ bone tissue engineering must be cancellous allogeneic bone because of the large surface area of cancellous bone and the exposed microscopic collagen strands on its surface, neither of which is present if cortical allogeneic bone is used.

Application. The application of these technologies is accomplished at the time of the bone grafting procedure, where the BMA or BMAC is mixed with the cancellous allogeneic bone and clotted with 0.5 ml of a mixture obtained by adding 5 ml of 10% calcium chloride to 5,000 units of either lyophilized bovine thrombin or recombinant human thrombin. It is this clotting that causes the cell adhesion molecules to bind to the allogeneic bone surface as well as releases the numerous growth factors from the bone marrow platelets and megakarcytes (i.e., three isomers of PDGF, VEGF, TGFb-1, TGFb-2,
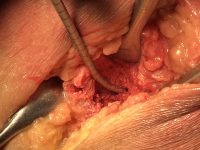
The surgeon then must cut the rhBMP-2/ACS wetted sponge that will deceptively appear like a wet bathroom tissue at this point into small squares (approximately 5 mm x 5 mm’s) and mix thoroughly into the clotted BMA/BMAC cancellous allogeneic bone composite (see Figure 9). This is done so that a nidus of bone regeneration will form throughout the volume of the graft and will consolidate to form a functional bone capable of growth, remodeling, and fusion to host bone and in the jaws acceptable for dental implants to replace teeth lost from tumor or trauma (see Figures 10 and 11).
|
|
The predictability of in-situ bone tissue engineering is due to its foundation on sound embryologic and wound healing principles. Its attraction for the surgeon is its simplicity and safety. Its value to the healthcare system is in its significant reduction in operating room time and hospitalization, and therefore cost to the healthcare system. Its greatest contribution to the individual patient is its reduction in pain, post-operative care, and morbidity, generating an early return to the workplace and to their families.