In a recent interview, Andreas Stange, VP, Medical and Health Services (MHS), Global at TÜV SÜD, noted, “When we used to talk about EU MDR and IVDR, we’d describe the regulations like a tsunami–a giant wave coming to swallow us.” Then, he said, we realized that the regulations are not a wave because a wave eventually goes down again, and these regulations will not go down.
“Instead, regulatory compliance is only going to become more important,” said Stange, “EU MDR and IVDR are here to stay.”
According to Stange, “The underlying difficulty is establishing a system that puts everything together in context.” Evidence, knowledge, and data ownership are often dispersed across companies by business unit, department, and area of expertise. Stange said that: “This siloed approach makes it difficult for manufacturers to tie all that together and understand how that should work; in the context of EU MDR and IVDR.”
Without a system or data infrastructure that can tie everything together in the context of the regulations, compliance is likely to remain challenging, plagued by numerous and costly inefficiencies, redundancies, and, most importantly, untold missed innovation opportunities.
The real problem, however, is not compliance. The real problem is embracing change that will drive innovation.
Therefore, driving innovation is both the core challenge and the single most significant opportunity that EU MDR and IVDR have created. Fundamentally, this inflection point for the diagnostics industry mainly comes down to evidence or data overload at a more fundamental level.
Clinical and Performance Evaluation as a Continuous Process
Embracing the concept of compliance as a continuous process
During a recent MedTech Intelligence (MTI) webinar entitled, Managing CERs and PERS Programmatically to Meet EU Regulatory Requirements, four panelists discussed some of the common evidence hurdles manufacturers face in meeting the EU MDR and IVDR requirements
The MTI webinar covered several important topics, including the literature review process, preparing submissions, working with notified bodies, and innovation related to facilitating better outcomes.
Throughout the discussion, some important themes emerged, including the clarity of telling a straightforward story, transparency of reasoning, and looking at the “totality of evidence.” But the one idea that came up with all four speakers was that clinical and performance evaluation is now a continuous process that must be integrated into a new, more holistic compliance approach.
According to MTI webinar speaker Bassil Akra, Ph.D. Founder & CEO at AKRA Team GmbH, the new regulations demand much more than identification, appraisal, and analysis of data that concludes: “Yes, my benefit-risk profile is acceptable.” The process doesn’t stop there; “This is a problem where a lot of people are not getting the message. It’s an The Post-Market Gambit ongoing continuous process.” He added, “You need to check your devices continuously.” For example, looking at the evidence and asking, “am I still meeting the acceptance criteria, the safety and performance expectations… is my device still safe and performing well?’”
Echoing Dr. Akra’s statement, speaker Sara Paquette, Ph.D., Manager, Medical Writing at Medtronic, called the process a “mindset shift.” She said that: “Under the new regulations, you go from an evaluation of the clinical data on the device to a continuous ongoing methodologically sound procedure.” This means “working that procedure out, determining what constitutes a methodologically sound process, and then [determining] how to implement that and maintain it, on a continuous level.” This mindset shift, she said, “is definitely something new.”
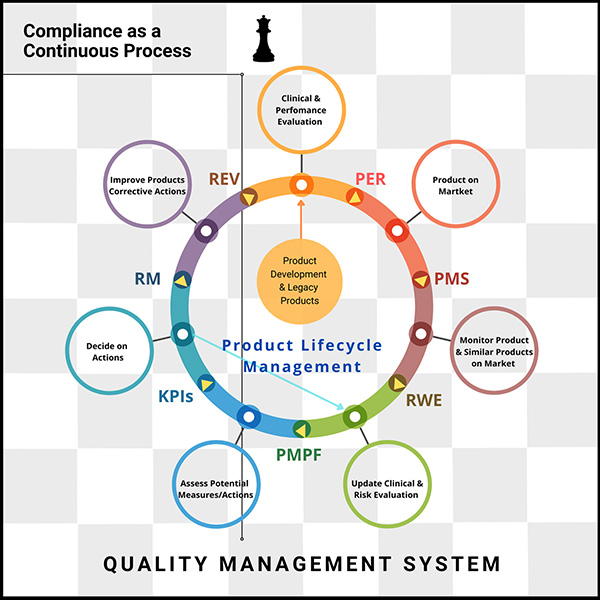
- Product Development and Legacy Products
- Clinical and Performance Evaluation
- Performance Evaluation Review (PER)
- Product on Market
- Post-Market Surveillance (PMS)
- Monitor Products and Similar Products on the Market
- Real-World Evidence
- Update Clinical and Risk Evaluation
- Post-Market Performance Follow-up (PMPF)
- Assess Potential Measures and Actions
- Monitor Key Performance Indicators (KPIs)
- Decide on Actions
- Ongoing Risk Management
- Improve Products and Take Corrective Actions
- Management Review and Decision-Making
* * *
This article is reprinted from “The Post-Market Gambit: After EU MDR and IVDR—A Systematic Compliance Framework for Driving MedTech Innovation,” Edited by Bassil Akra, Ph.D. Download the full 60-page eBook here for free or purchase it on Amazon.com.