It is a troubling reality that diabetes will continue to be a prevalent problem in the United States and throughout the world. While advocacy groups such as the American Diabetes Association and the International Diabetes Federation (IDF) work to raise awareness among the general population and guide those living with the disease, medical device companies are trying to keep up with patient demand for more control over their condition.
“According to a study by the IDF, only a very small number of patients, less than 12%, actually reach their therapy targets. This is partly due to access to decent care, but apart from this, the motivation of patients to take responsibility and actively manage their disease is another important aspect,” says Luc Vierstraete, global head of Roche Diabetes Care. Insulin pumps and continuous glucose monitoring (CGM) technology, which more effectively helps patients manage insulin delivery, are aiding in the reduction of death rates as a result of hyperglycemia, lower-limb amputation rates, and kidney failure by improving blood glucose control.

Diabetes Tech Considerations
The increasing number of people living with insulin-dependent diabetes influences how companies develop technologies in several ways. “Factors such as the ability to offer real innovation to help people with diabetes effectively manage their disease and avoid long-term complications as well as markets with limited reimbursement, access to care in emerging markets, and production capacity for large-scale availability play an important role,” says Vierstraete. Companies must be able to refine technologies that help improve medical outcomes and offer accessibility to a broad range of patients with diabetes while operating in a market of increased pressures due to reimbursement challenges, pricing declines and more stringent regulatory requirements, according to Vierstraete. The market is highly competitive, with Medtronic Diabetes, Roche Diabetes Care, Animas Corp., Tandem Diabetes Care, Inc., and Smiths Medical being among the key players.
Although device companies commonly consider human factors into product design, this is especially important for technologies in the diabetes space, as much more interaction occurs between the patient and the product, whether it’s a device connection, touchscreen interface or a mobile app. Tandem Diabetes Care is taking its user-centric approach to another level by offering insulin pumps that range from the largest capacity pump approved by FDA (the t:flex) to the smallest and thinnest pump on the market (the t:slim) to the only FDA-approved touchscreen pump with CGM (the t:slim G4). The company also has a t:simulator app that integrates with an iPhone or iPad.
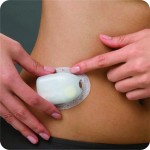
Then there’s the departure from the conventional: The OmniPod, an insulin management system manufactured by Insulet Corp. Considered part of the insulin pump market, the OmniPod is the only tube-free pump on the market. While a traditional pump sits on the user’s waist with a tube attached to a catheter that injects into the abdomen, the OmniPod is a disposable automatic patch pump (about the size of a large strawberry) with an adhesive backing to enable discrete placement anywhere on the body. The device provides 72 hours of continuous insulin delivery, which the patient wirelessly controls via the personal diabetes manager (PDM).
“Through market research, patients have said they would like to be able to control their insulin pump and their OmniPod from their phone,” says Shacey Petrovic, chief commercial officer at Insulet. “While technically that is feasible, we sell in a highly regulated market and so FDA and other agencies involved in improving these devices need to catch-up with the consumer. Our challenge is to deliver as much mobile experience and value as we can for our consumer in a heavily regulated marketplace.”
The other obvious challenge is the protection of patient privacy and data security. Roche’s emminens eConecta web-based platform is a secure system designed to enable confidential information exchange for patients and healthcare professionals, combining the analysis and compilation of data on blood glucose, insulin and other clinical parameters for monitoring diabetes in the patient history. In June Medtronic received FDA clearance for its MiniMed Connect, a device that securely sends pump and sensor glucose data from a patient’s pump to the app or web display. Small enough to fit on a keychain, the product shows real-time sensor glucose and insulin information and provides secure remote monitoring by patient caregivers or loved ones.
Mobile Data Integration Empowers Patients
A big trend in the diabetes market is the integration of CGM data with insulin pump monitoring data, much of which is occurring via collaboration. “From a technical perspective, there are a lot of opportunities in this market for partnerships [between]—the insulin [pump] manufacturers and the continuous glucose manufacturers, with Dexcom being the market leader,” says Petrovic. Insulet recently announced a partnership with Dexcom to integrate the company’s latest sensor G5 data into Insulet’s next-generation PDM platform. Insulet plans to leverage mobile technology to make the experience more meaningful for patients. The project is currently in the development stage, but Petrovic anticipates the platform to hit the market late next year or early 2017. “An interesting dynamic in this marketplace is that patients are consumers,” she says. “They’re using these devices every day at every hour, so they’re looking for ways that mobile technology and digital technology can make their lives easier.” Apps that collect patient data and provide visualization of that data can empower patients to make more informed choices about how they deliver their insulin.
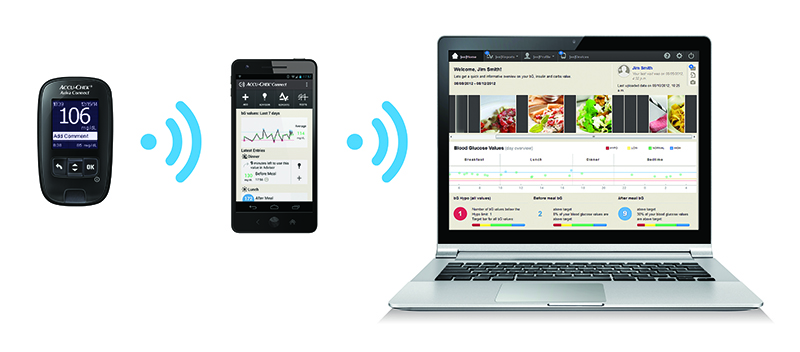
Although it has provided a dramatic change to the way in which patients manage their condition, the integration of software and electronics has also introduced a new set of challenges to technologies in the diabetes market. “This translates into much greater complexity of the systems, more dependency on external players and substantial additional effort in the areas of validation and regulatory management for us as well as for the authorities,” says Vierstraete. “On the other hand, the link to consumer electronics also poses a great opportunity for us to develop new ways of addressing customer needs, whether they are patients, doctors or insurance companies, and provide eHealth solutions that can greatly enhance our customers’ possibilities to manage diabetes in an effective and efficient way.”
Vierstraete points to trends in e-Health and more sophisticated mobile apps, better interoperability and the miniaturization of devices as the drivers in growing the next generation of diabetes management products. These capabilities will provide more effective doctor-patient communication and peer-to-peer-communication, increasing patient motivation and engagement. “One short- to mid-term innovation we will also see in diabetes is the improved accuracy of CGM devices, which is in particularly important in the area of sensor-augmented pumps when the CGM signal is used to steer the pump,” says Vierstraete.
 Glucose Test Strips: Materials Change the Game
Glucose Test Strips: Materials Change the Game
With a prick of a finger and a drop of blood on a test strip, patients can test whether their blood sugar is too high or too low. A lot of the focus surrounding diabetes technology involves the devices themselves, but what about the components? Materials such as adhesives and hydrophilic films play a pivotal role, from holding a test strip together to controlling accuracy of results. “The adhesive plays a couple different roles; it has to hold the whole strip together,” says Raymond Kenney, senior product development specialist in the display materials and systems division at 3M. “But because the adhesive is die cut through to create the capillary channel to measure the volume of blood that lifts up into the strip, the caliper control in the adhesive is extremely important.”
The challenge with adhesives, whether a transfer adhesive or a tape, lies in providing a consistent thickness throughout the entire tape construction. Achieving precise caliper control results in an increase in accuracy of the volume of the chamber, which increases the accuracy of the final result for the concentration of glucose in the volume of blood.
Manufacturers of blood glucose monitors are seeking cost-effective hydrophilic films that perform with more accuracy without a delay from the time the patient’s blood hits the strip to the blood glucose reading. “The hydrophilic film works by lowering the surface tension of blood as it comes into the strip so that you get accurate speed and volume out of the test results,” says Kenney.
One of the big design issues with materials that go into a blood glucose strip is managing the small amount of contamination (at a parts per billion level) that can occur. “Limiting the amount of contamination is especially important in the hydrophilic film, because a lot of the competition in the market right now still use the old technology of dumping a bunch of surfactant on the surface of the film in order to lower the surface tension,” says Kenney, adding that all of the surfactant goes into the blood, resulting in a lot of contamination. Seeking a solution to the issue, 3M recently commercialized a hydrophilic film that has no leachate into the blood while providing fast and accurate wicking.
The Holy Grail of Insulin Therapy: The Artificial Pancreas
The first steps toward the artificial pancreas are realized in the integration of the continuous glucose monitoring information. “The artificial pancreas means different things to different people. It essentially is aiming for a hybrid or completely closed loop system where the CGM monitors glucose in the patient, sends that information to the pump and the pump automatically delivers insulin based on the continuous glucose reading from the monitor,” says Petrovic.
From the Bionic Pancreas (also known as the iLet) to a “dual-hormone” artificial pancreas numerous devices and approaches are being examined worldwide to determine how patients best respond to various methods of insulin therapy.
“To be able to correlate what fluid you’re measuring to what you’re actually measuring in the blood—that’s the real challenge,” says Kenney. “All the separate technologies exist. We have infusion pumps that can deliver an accurate dose of insulin to the blood; we have test strips that can accurately measure glucose concentration. Getting some type of closed feedback on it through measuring an alternative fluid is a big challenge. [If achieved], that would be a winner in this industry.”
Behind DiabetesThere are three forms of diabetes:
|
|
Driving Patient Adherence
Regardless of the product approach, the goal of technologies in diabetes treatment and management is to reduce the patient burden in calculating and delivering the correct amount of insulin at the right time. But this is only half the battle. Patient adherence is a critical part of long-term success in achieving glycemic control. This is specifically an issue in the pediatric population in which more than 200,000 U.S. children have type 1 diabetes. Driving adherence requires a two-pronged approach. First: Make the devices as easy to use as possible. “By and large, [insulin] pumps are incredibly complex and very challenging devices to use,” says Petrovic. “There are multiple steps to prime a traditional pump.” Second is the digital aspect: Provide more meaningful data to patients, caregivers and clinicians. “An informed patient is an empowered patient, and that patient can take control of [his or her] compliance and outcomes,” says Petrovic.