

The technology platform has already gone global and has the potential to be a true breakthrough in screening and detecting several types of cancer.

The technology platform has already gone global and has the potential to be a true breakthrough in screening and detecting several types of cancer.

One town in Pennsylvania is preparing for the Papal visit by equipping its various sites with defibrillators.

FDA issues safety communication alert for Hospira’s Symbiq Infusion System.

From glucose monitors to heart monitors, the mobile technology market for medical products continues to expand.
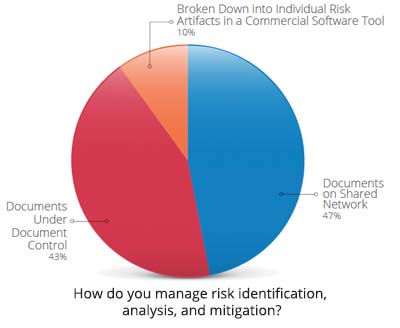
With the increased complexity of devices, a streamlined approach to managing product development risks and documenting compliance is challenging but perhaps more important than ever.

Manufacturers that make custom devices must move beyond conventional engineering practices to an automated and collaborative engineer-to-order process.
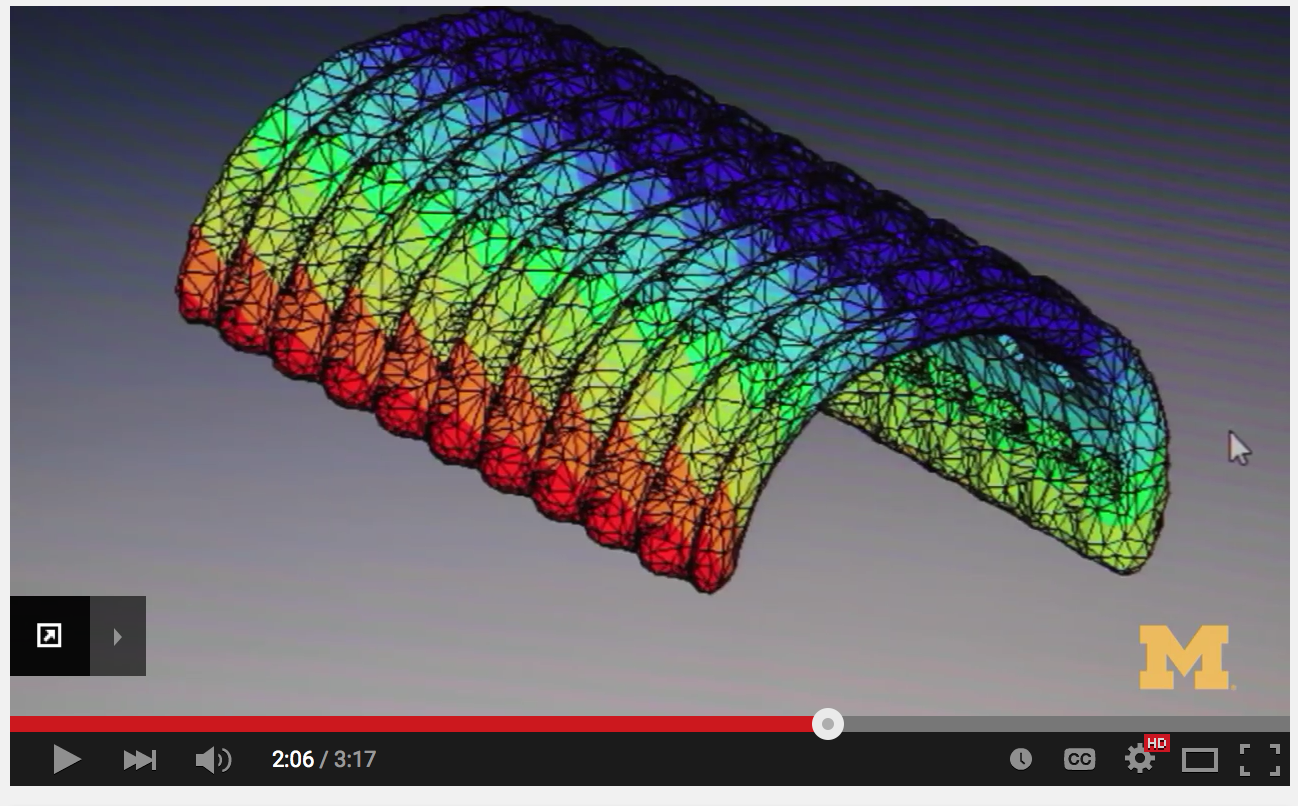
For 3D printing to thrive in the medical device space, the use of biomaterials must be expanded.
As additive manufacturing comes to the forefront as a disruptive technology, industry continues to speculate about how liability will be shared.
This technology is poised to deliver cost benefits and advance innovation in manufacturing, and companies will have to focus on replacing old protocols relying on extensive up-front testing and validation of traditional production tools, and understand steps needed to satisfy their quality requirements in the future, describes a new McKinsey report.
Popularity of smartphone applications, or apps, has increased explosively. Among the implemented functionality, more and more medical device features have started to creep in. In this article, we discuss when an app is simply fun and when it needs to be regulated as a medical device.