

Smart process automation promises to cut complexity and transform product traceability in the medical device industry. However, its potential relies 100% cent on having good, reliable data on which to draw.

Smart process automation promises to cut complexity and transform product traceability in the medical device industry. However, its potential relies 100% cent on having good, reliable data on which to draw.

Timely compliance will ensure that your products will not be considered misbranded.

With the Covid-19 pandemic increasingly forcing healthcare testing, diagnosis and treatment online, the promise of augmented and virtual reality is drawing deepening interest in Asia’s medtech market.

Sources of reliable data to continuously support claims of compliance by manufacturers will be in high demand in the future.
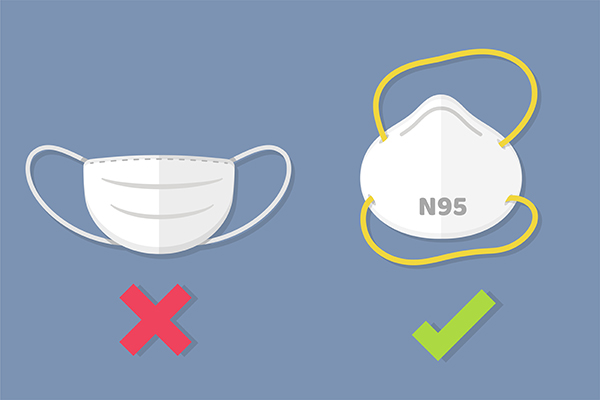
As the saying goes, if it looks too good to be true, it probably is.

Taking a bare-minimum approach to meeting the eIFU requirements of EU MDR could mean missing an opportunity to improve transparency in healthcare. Here’s how to use eIFU to provide stakeholders with greater confidence and clarity in medical devices.

AdvaMed has reported that its member companies have provided $26.8 million in medical supplies, along with $4.1 million in cash donations.

The enforcement operation kept nearly 500 shipments of illegal drugs and combination devices from the hands of consumers.

The state’s attorney general said J&J put its profits “ahead of the health of millions of women”.

The latest regulations for human and veterinary medicinal products, as well as medical devices, include the mandate to set up databases with detailed information about available products. These databases must be realized and implemented in the near future, and require a concerted effort now if tangible real-world benefits are to follow.