

Outdated policies and regulations threatens to bring progress to a standstill, restrict vital telehealth access to millions of Americans, and exacerbate health inequities.

Outdated policies and regulations threatens to bring progress to a standstill, restrict vital telehealth access to millions of Americans, and exacerbate health inequities.

You’d think there’s not much to the concept of an alarm. A warning sounds in a room, or a red light flashes, and it has your attention. You know something must be wrong. But in a setting where a cacophony of alarming lights and sounds beset healthcare workers on a regular basis, these essential systems become increasingly easy to miss or ignore.

Patient-administered healthcare is one of the fastest-growing segments in the medtech industry. When the patient becomes the operator, usability requirements are vastly different than those of trained clinicians, which elevates considerations in the design process.

With the ever-increasing adoption of connected devices, the agency is emphasizing the need for effective cybersecurity.

Blockchain technology helps support the goal of decentralizing the healthcare sector and providing patients with the information they deserve about their own healthcare data.
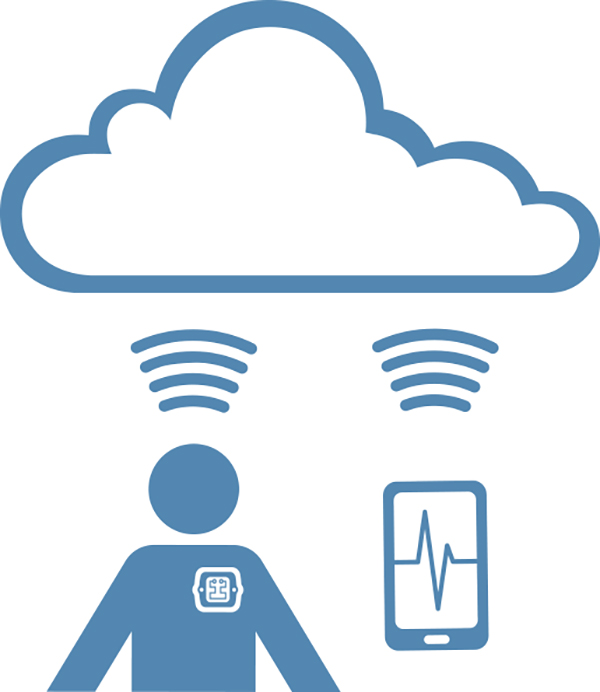
Experts will explore how digital technologies have opened up new opportunities for patients, providers and medtech manufacturers.

The total $8.4 billion fiscal year 2023 budget request is 34% higher than the agency’s 2022 appropriated funding level.

The combination of medtech progress with strides in modern healthcare interoperability will enable preventive care in unprecedented ways.

New changes mean new challenges, but global harmonization could make things easier for device manufacturers in the long run.

2022 is now upon us, hopefully marking the end of a very disruptive couple of years. Global challenges, from supply chain shortages to remote services, have driven digital innovation and change in the way we work across all sectors. The medical device industry is no different and going forward, having learned from the experiences of 2021.